Nutrient Cycles in Nature
The nutrient cycle refers to the movement and exchange of organic and inorganic matter to support the production of living organisms. It examines how essential molecules in an ecosystem are transferred. Like energy, nutrients are never lost from the cycle; they are stored in different forms, such as fossil fuels, living organisms, or Carbon (IV) Oxide (CO2). Microorganisms play a critical role by feeding on dead material and converting complex organic molecules into simpler ones.
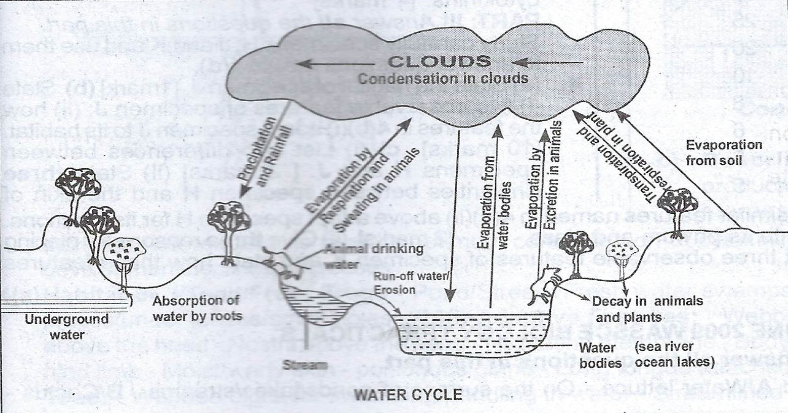
The Water Cycle
The water cycle describes the continuous movement of water above, on, and below the Earth’s surface. Key processes in the water cycle include:
- Evaporation: Water changes from its liquid state to vapor, occurring over water bodies like oceans, streams, and lakes, as well as surfaces like soil, vegetation, or rocks. The vapor rises into the atmosphere.
- Condensation: Water vapor cools and changes into liquid, forming fog, dew, or clouds. When these grow heavy, they fall back to the Earth as precipitation.
- Precipitation: Water droplets or ice particles fall to the Earth’s surface due to gravity. Precipitation may infiltrate the soil, flow as runoff, or evaporate back into the atmosphere.
- Interception: Water movement is interrupted when absorbed by vegetation, stored in puddles, or caught by natural formations like furrows and streams.
- Infiltration: Water slowly penetrates the soil, influenced by factors like soil texture and structure. It’s stored and may later evaporate.
- Percolation: Water flows through soil and rock, driven by gravity and capillary forces, eventually becoming groundwater that moves horizontally beneath the Earth’s surface.
- Transpiration: Plants release water vapor into the atmosphere through leaf openings during the day, connecting soil moisture to the atmosphere.
- Runoff: Excess water from precipitation flows overland to water bodies or infiltrates the soil.
- Storage: Water is stored in the atmosphere, on the Earth’s surface, or underground as part of the hydrological cycle.
The Carbon Cycle
The carbon cycle describes how carbon moves through the atmosphere, living organisms, and the environment:
- Carbon (IV) Oxide (CO2) in the atmosphere is used by plants for photosynthesis to create carbohydrates, removing carbon from the atmosphere.
- When animals consume plants, carbon is transferred from plants to animals.
- Respiration by plants and animals releases CO2 back into the atmosphere.
- When plants and animals die, decomposers break down organic matter, releasing CO2 during the decay process, maintaining the cycle’s balance.
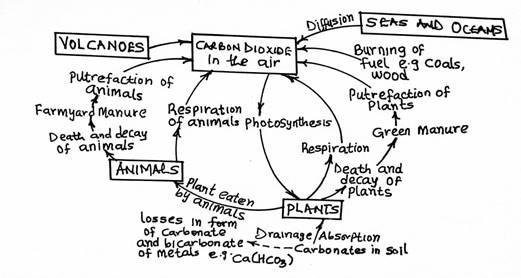
Human activities, such as burning fossil fuels, disrupt this balance by adding excess CO2 to the atmosphere.
Importance of Carbon
- Plants use CO2 to produce food during photosynthesis.
- Carbon is a key building block of all organic matter.
- It helps maintain the atmospheric CO2 levels necessary for a balanced environment.
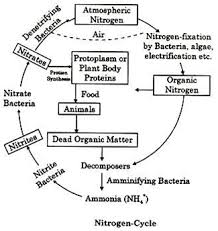
The Nitrogen Cycle
The nitrogen cycle involves processes that circulate nitrogen between living organisms and the environment. Nitrogen exists as amino acids and proteins in living organisms and as gaseous nitrogen or inorganic salts like nitrates in the soil. Key processes in the nitrogen cycle include:
- Nitrogen Fixation: Nitrogen gas from the air is converted into nitrogen compounds like nitrates and ammonium, either by nitrogen-fixing bacteria or lightning.
- Nitrification: Ammonia in the soil is converted into nitrates by nitrifying bacteria, which plants absorb to produce proteins.
- Denitrification: Nitrogen compounds are converted back into nitrogen gas by bacteria, returning it to the atmosphere. This process occurs in small amounts.
- Decay: Dead organisms and waste materials release organic nitrogen compounds, which decomposers convert into ammonia and ammonium compounds in the soil.
- Lightning Fixation: Lightning combines nitrogen and oxygen to form nitrogen oxides, which enter the soil and form nitrates.
Importance of Nitrogen
- Plants absorb nitrogen from the soil as nitrates or ammonium salts and use it to make proteins and other nitrogen containing compounds needed for proper growth and development.
- Animals obtain their nitrogen in form of proteins in plants and use it to build new cells and repair worn out tissue.
- Symbiotic bacteria in the root nodules of leguminous plants synthesize organic substances (protein) from atmospheric nitrogen.
- Some soil bacteria obtain energy by oxidizing ammonium salts and nitrates.
Oxygen cycle
The oxygen cycle elaborates how oxygen circulates in various forms through nature. Oxygen occurs freely in the air, trapped in the earth crust as chemical compounds, or dissolved in water. Oxygen in the atmosphere is about 21%, and it is the second most abundant gas after nitrogen. It is mostly utilized by living organisms, especially man and animals in respiration. Oxygen is also the most common element of human body. The circulation depends on the various activities on Earth.
Oxygen Cycle Steps:
- Atmosphere: Only a small percentage of the world’s oxygen is present in the atmosphere, only about 0.35 %. This exchange of gaseous oxygen happens through Photolysis. Photolysis is the process by which molecules like atmospheric water and nitrous oxide are broken down by the ultraviolet radiation coming from the sun and release free oxygen.
- Biosphere: The exchange of oxygen between the living beings on the planet, between the animal kingdom and the plant kingdom. The exchange of oxygen in the biosphere is codependent on the Carbon cycle and hydrogen cycle as well. It mainly occurs through 2 processes
- Photosynthesis: The process by which plants make energy by taking in Carbon (IV) Oxide from the atmosphere and give out oxygen
- Respiration: The process by which animals and humans take in oxygen from the atmosphere and use it to break down carbohydrates and give out carbon dioxide.
- Lithosphere: part of the planet containing most of the oxygen content through biomass, organic content and mineral deposits. These deposits are formed when free radical elements were exposed to free oxygen and over time they form silicates and oxides. This trapped oxygen is released back due to several weathering processes. Also, animals and plants draw nutrient materials from the from the lithosphere and free some of the trapped oxygen.
- Hydrosphere: Oxygen dissolved in water is responsible for the sustenance of the aquatic ecosystem present beneath the surface. The hydrosphere is 33% oxygen by volume present mainly as a component of water molecules with dissolved molecules including carbonic acids and free oxygen.